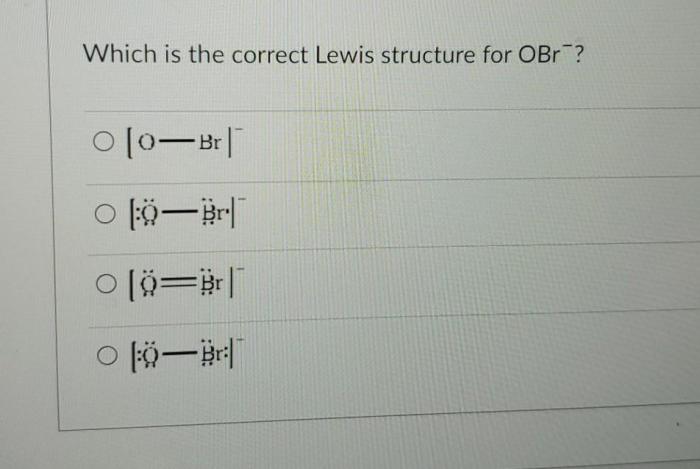
Which is the correct Lewis structure for OBr? This question sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. Prepare to embark on a journey of scientific exploration as we delve into the intricacies of OBr’s molecular structure, uncovering its bonding characteristics, resonance structures, polarity, and more.
The Lewis structure of a molecule provides a visual representation of the arrangement of its atoms and the distribution of its electrons. For OBr, the correct Lewis structure is crucial for understanding its chemical behavior and properties. This exploration will provide a comprehensive understanding of OBr’s molecular makeup, enabling a deeper comprehension of its role in various chemical processes.
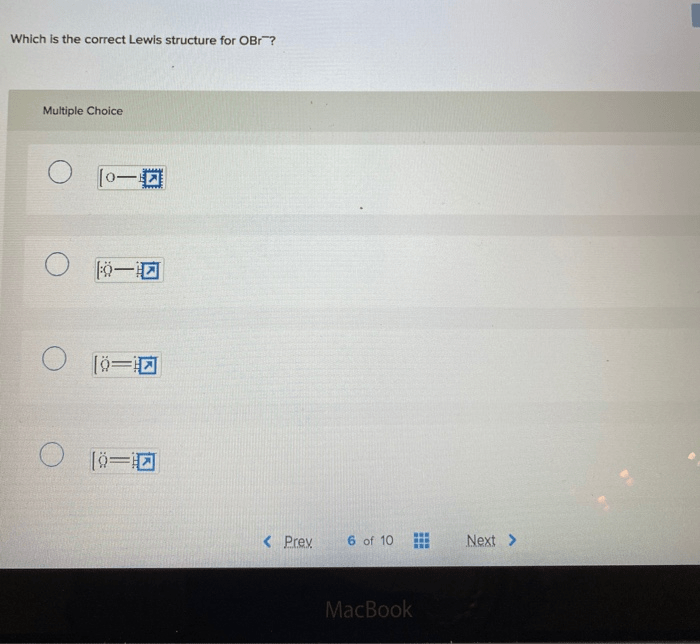
Lewis Structure of OBr
OBr is a diatomic molecule composed of oxygen and bromine atoms. The correct Lewis structure for OBr is:“`:O-Br:“`In this structure, the oxygen atom has two lone pairs of electrons, while the bromine atom has one lone pair. The oxygen and bromine atoms are bonded by a single covalent bond.
Molecular Geometry of OBr
According to VSEPR theory, the molecular geometry of OBr is linear. This is because the oxygen atom has two lone pairs of electrons, which repel each other and push the bromine atom away from the oxygen atom. The bond angle between the oxygen and bromine atoms is 180 degrees.
Resonance Structures of OBr
OBr does not have any resonance structures. This is because the oxygen atom has two lone pairs of electrons, which cannot be delocalized.
Polarity of OBr: Which Is The Correct Lewis Structure For Obr
OBr is a polar molecule. This is because the oxygen atom is more electronegative than the bromine atom. As a result, the electrons in the O-Br bond are pulled towards the oxygen atom, creating a partial negative charge on the oxygen atom and a partial positive charge on the bromine atom.
Bonding in OBr
The bond in OBr is a covalent bond. This is because the oxygen and bromine atoms share electrons in order to form a stable molecule. The oxygen atom contributes two electrons to the bond, while the bromine atom contributes one electron.
The bond is formed by the overlap of the oxygen atom’s 2p orbital and the bromine atom’s 4p orbital.
Properties of OBr
OBr is a toxic gas with a pungent odor. It is denser than air and is soluble in water. OBr is a powerful oxidizing agent and can react violently with many other substances.
Expert Answers
What is the hybridization of the oxygen atom in OBr?
sp 3
How many lone pairs are present on the bromine atom in OBr?
Two
Does OBr have any resonance structures?
No