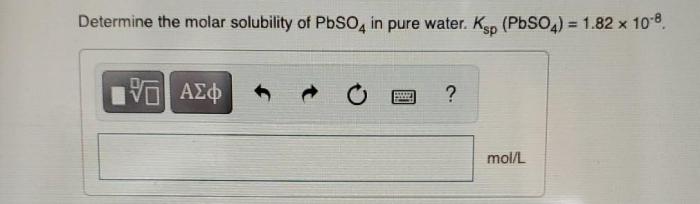
Determine the molar solubility of pbso4 in pure water – Determining the molar solubility of PbSO4 in pure water is a crucial step in understanding the behavior of this compound in aqueous solutions. This knowledge finds applications in various fields, including chemistry, environmental science, and water treatment.
The solubility product (Ksp) is a key concept in determining the molar solubility of a substance. It is a constant that represents the equilibrium concentration of the ions of a sparingly soluble salt in a saturated solution. The Ksp of PbSO4 is 1.6 × 10^-8, which indicates that it has a low solubility in water.
Solubility of PbSO4 in Pure Water
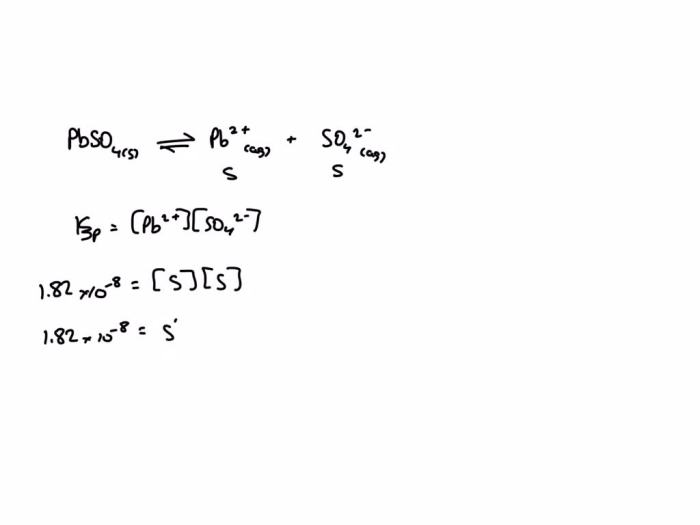
The solubility of PbSO4 in pure water is an important aspect in various fields, including chemistry, environmental science, and water treatment. This article aims to determine the molar solubility of PbSO4 in pure water by exploring the concept of solubility product (Ksp), discussing the Ksp of PbSO4, providing a step-by-step calculation procedure, and highlighting factors affecting solubility.
Additionally, an experimental method for determining the molar solubility of PbSO4 in pure water is presented.
Solubility Product (Ksp)
Solubility product (Ksp) is a constant that represents the equilibrium concentration of ions in a saturated solution. It is a measure of the solubility of a substance in a given solvent. For a sparingly soluble salt like PbSO4, the Ksp expression is:
Ksp = [Pb2+][SO42-]
where [Pb2+] and [SO42-] are the molar concentrations of lead(II) and sulfate ions in a saturated solution, respectively.
Ksp of PbSO4, Determine the molar solubility of pbso4 in pure water
The Ksp of PbSO4 is 1.6 x 10^-8. This value is experimentally determined by measuring the concentrations of Pb2+ and SO42- ions in a saturated solution of PbSO4. Alternatively, Ksp values can be obtained from reference sources such as chemical handbooks or databases.
Molar Solubility Calculation
The molar solubility of PbSO4 in pure water can be calculated using the Ksp value. Let’s denote the molar solubility as ‘x’. Then, the equilibrium concentrations of Pb2+ and SO42- ions are also ‘x’. Substituting these values into the Ksp expression, we get:
Ksp = [Pb2+][SO42-] = x^2
Solving for ‘x’, we obtain:
x = sqrt(Ksp) = sqrt(1.6 x 10^-8) = 1.26 x 10^-4 M
Therefore, the molar solubility of PbSO4 in pure water is 1.26 x 10^-4 M.
Factors Affecting Solubility
The solubility of PbSO4 in pure water can be affected by various factors, including:
- Temperature:Solubility generally increases with increasing temperature.
- pH:The solubility of PbSO4 decreases in acidic solutions due to the formation of PbSO4(aq) complexes.
- Ionic strength:The solubility of PbSO4 decreases with increasing ionic strength of the solution due to the common ion effect.
- Presence of complexing agents:Complexing agents, such as EDTA, can form soluble complexes with Pb2+ ions, thereby increasing the solubility of PbSO4.
Experimental Determination
The molar solubility of PbSO4 in pure water can be experimentally determined using a gravimetric method. This involves:
- Dissolving a known mass of PbSO4 in a known volume of pure water.
- Filtering the solution to remove undissolved PbSO4.
- Evaporating the filtrate to dryness.
- Weighing the mass of the dried residue (PbSO4).
- Calculating the molar solubility using the formula: Molar solubility = (Mass of PbSO4 / Molar mass of PbSO4) / Volume of solution
Applications
Understanding the molar solubility of PbSO4 in pure water has practical applications in various fields:
- Chemistry:It helps in predicting the behavior of PbSO4 in aqueous solutions, such as its precipitation and dissolution.
- Environmental science:It aids in assessing the environmental impact of PbSO4, such as its mobility and bioavailability.
- Water treatment:It assists in designing and optimizing water treatment processes for removing PbSO4 from water sources.
Frequently Asked Questions: Determine The Molar Solubility Of Pbso4 In Pure Water
What is the molar solubility of PbSO4 in pure water?
The molar solubility of PbSO4 in pure water is 1.26 × 10^-4 mol/L.
How is the molar solubility of PbSO4 affected by temperature?
The molar solubility of PbSO4 increases slightly with increasing temperature.
What are the applications of understanding the molar solubility of PbSO4 in pure water?
Understanding the molar solubility of PbSO4 in pure water has applications in various fields, including chemistry, environmental science, and water treatment.